Journal of Animal Research: v.10 n.2, p. 195-198. April 2020
DOI: 10.30954/2277-940X.02.2020.5
Detection of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in Desi Chickens in Andhra Pradesh
ABSTRACT
A total of 150 cloacal swabs were collected from desi chickens, 217 Enterobacteriaceae isolates were identified. The phenotypic antimicrobial resistance among Enterobacteriaceae was studied for 14 selected antibiotics by disc diffusion method. The selection of antibiotics was based on usage of antibiotics in commercial poultry farms and also based on priority of critically important antibiotics in humans. All Enterobacteriaceae isolates were subjected to multiplex PCR - I and II for detection of blaTEM, blaSHV and blaOXA genes and blaCTX-M, group 1 and 2 genes. Predominant β- Lactamase genes in gut microbiota of desi chicken include blaTEM (90.55%) followed by blaCTX-M group I (25.86%) and blaSHV (9.44%) genes. All the samples were found to be negative for blaOXA and blaCTX-M group 2 genes.
Keywords: Desi chickens, Enterobacteriaceae, blaCTX-M, blaSHV, and blaTEM genes
Extended-spectrum beta-lactamase (ESBL) producers are Gram-negative bacteria that produce enzymes that bestow resistance to most beta-lactam antibiotics like penicillin’s, cephalosporins and the monobactam. These ESBL producers have been noticed mainly in the Enterobacteriaceae family of bacteria which may harbour several antibiotic resistance determinants making treatment of infections caused by these pathogens more difficult. Extended-spectrum beta-lactamase producers have a complex epidemiology; the most prominent bacteria involved include E. coli and K. pneumoniae whose reservoirs comprise the environment (soil and water), wild animals, farm animals, food and pets (Sharif et al., 2017a). Extended-spectrum beta-lactamase-producing bacteria are frequently resistant to many antimicrobial agents usually recommended for the treatment of infections such as gentamicin, fluoroquinolones and trimethoprim- sulfamethoxazole. This leads to serious challenges in the treatment of ESBL- Enterobacteriaceae infections because the bacterial plasmids may harbour several antibiotic resistance determinants. Heavy usage of antibiotics in commercial poultry farms has been reported to be a risk factor in the acquisition of ESBL-producing organisms (Sailu et al., 2017). These organisms may enter into the environment and human food chain. Hence, the present study was planned with the objective to isolate the gut microbiota and to study their antimicrobial properties.
How to cite this article: Divya, M., Sreedevi, B., Chaitanya, R.K. and Srilatha, Ch. (2020). Detection of extended-spectrum beta-lactamase-producing enterobacteriaceae in desi chickens in Andhra Pradesh. J. Anim. Res., 10(2): 195-198.
MATERIALS AND METHODS
Sample collection
A total of 150 cloacal swabs were collected from different villages in and around Tirupati, A.P. which include Chittoor, Venkatagiri, Tirupati, Nagalapuram, Pallam, Vampalli, B. Kandriga and Kalahasti.
Isolation and identification of bacteria
The samples were subjected to bacterial isolation, Divya et al. biochemical characterization with reference to Enterobacteriaceaeas per the standard protocols.
Antibiotic Sensitivity Test (ABST)
A total of 14 antibiotic discs were selected in the present study. The discs used include ampicillin, bacitracin, cefotaxime, chloramphenicol, ciprofloxacin, colistin, doxycycline HCL, gentamicin, enrofloxacin, vancomycin, furazolidone, nitrofurazone, virginomycin and tylosine. ABST was carried out using standard protocols. The selection of antibiotics was based on usage of antibiotics in commercial poultry farms and also based on priority of critically important antibiotics in humans. Inhibition zone diameters were interpreted according to CLSI (2014) guidelines (M100-S24).
DNA Extraction
DNA extraction was carried out by boiling and snap chilling method as described by Rao (2009) with minor modifications.
Polymerase Chain Reaction (PCR)
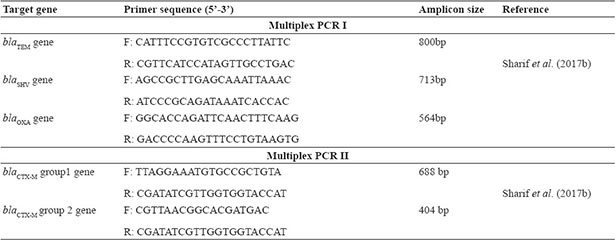
Multiplex PCR I and II were standardised and cycling conditions include initial denaturation at 94 ºC for 10 minutes. 30 cycles of denaturation at 94 ºC for 40 seconds, annealing at 60 ºC for 40 seconds, elongation at 72 ºC for 1 minute and final elongation at 72 ºC for 7 minutes and hold at 4ºC. Oligonucleotide primers used and their respective amplicon sizes were given in Table 1.
RESULTS AND DISCUSSION
Phenotypic antibiotic resistance in Enterobacteriaceae
In this study, a total of 217 isolates with reference to the family Enterobacteriaceae were obtained from the faeces of desi chicken. 130 (59.9%) were characterized as E.coli, 42 (19.35%) were characterized as Salmonella spp. and 45 (20.73%) isolates were characterized as Klebsiella spp. Hundred percent resistance was recorded against bacitracin, colistin, furazolidone, nitrofurazone, vancomycin, virginomycin and tylosine. 70.96, 58.52, 30.8, 26.72, 22.5 17.5 and 16.1% resistance was observed against Doxycycline Hcl, ampicillin, ciprofloxacin, cefotaxime, chloramphenicol, enrofloxacin and gentamicin respectively.
Even though, antibiotics are not used in desi chicken, they found to harbour resistant gut microflora. Antibiotic resistance was also reported in desi chicken by other workers. Kakkar et al. (2017) tested samples from backyard poultry in New Delhi. High level of resistance was reported to chloramphenicol, ciprofloxacin, gentamicin, levofloxacin, norfloxacin and oxytetracycline. Ibrahim, (2017) tested poultry cloacal swabs from Sudan and reported 60% resistance to enrofloxacin, 40, 20, 10 and 5% resistance to tetracycline, ciprofloxacin, gentamicin and colistin respectively. The samples which were found to be resistant to ampicillin and cefotaxime in ABST were selected and further screened for the presence of beta lactamase genes.
Table 1: Primers used for multiplex PCR I and II for the detection of beta lactamasegenes

Multiplex PCR I and II for the detection of beta lactamase genes
A total of 127 isolates showing phenotypic resistance to ampicillin were selected. The DNA extraction was carried out and the isolates were tested for the presence of blaTEM, blaSHV and blaOXA genes. Out of 127 (85 E.coli, 18 Klebsiella and 16 Salmonella), 103 (81.10%) isolates were found positive for the presence of blaTEM gene and 2 (1.57%) samples were found to be positive for the presence of blaSHV gene alone and 10 (7.87%) samples were found to possess both blaTEM and blaSHV genes (Fig. 2). None of the samples harboured blaOXA gene. Out of 115 blaTEM PCR positive samples, 85 (73.91%) belonged to E.coli, 14 (12.17%) were Klebsiella spp. and 16 (13.91%) were Salmonella spp. Out of 12 blaSHV PCR positive samples, 8 (66.66%) belonged to E.coli and 4 samples (33.33%) belonged to Klebsiella spp (Fig. 1).
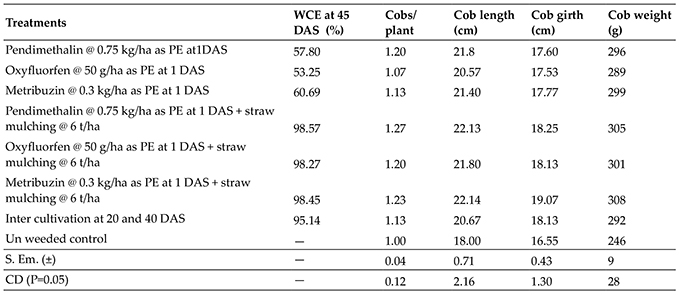
Fig. 1 Occurrence of tet A, blaTEM, blaSHV, blaOXA and blaCTXM Group I and II genes in enteric bacteria of desi chicken
A total of 58 isolates showing phenotypic resistance to cefotaxime were selected. The DNA extraction was carried out and the isolates were tested for the presence of blaCTXM Group 1 and Group 2 genes. Out of 58 (20 E.coli,
14 Klebsiella and 16 Salmonella), 15 (25.86%) samples were found to be positive for the presence of blaCTXM group I gene (Fig 3). None of the samples harboured Group 2 gene. Out of 15blaCTXM Group II PCR positive samples, 10 (66.66%) belonged to E.coli, 3 (20%) were Klebsiella spp. and 2 isolates (13.33%) were Salmonella spp (Table 2).
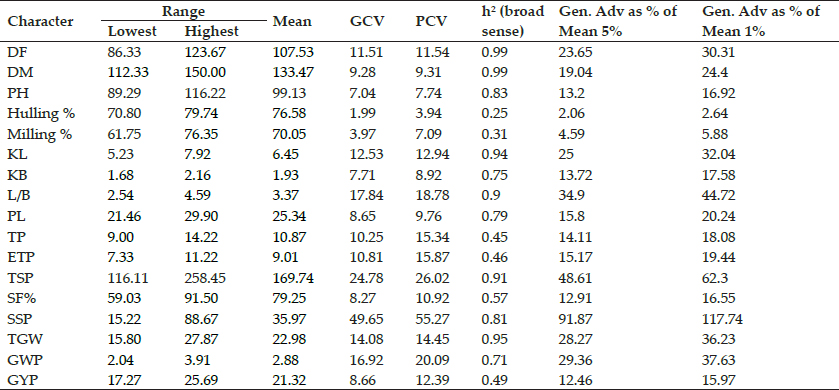
Fig. 2: Detection of Multiplex PCR I (blaTEM, blaSHV and blaOXA) genes in Enterobacteriaceae of desi chicken
Lane M: Molecular weight marker (100bp); Lane 1 : Known DNA standard carrying bla TEMand bla SHVgenes; Lane 2 to 6 : Desi chicken microbiota carrying bla TEM and bla SHVgene; Lane 7: Negative control.
Presence of ESBL genes were reported from desi chicken in other studies. Hasan et al. (2012) isolated 66 E.coli from desi chicken in Bangladesh. Out of 66, 36 E.coli harboured ESBL genes. 34 of them belonged to the blaCTX-M-1 group and 2 of them belonged to blaCTXM-9 group. Combinations of blaCTX-M-1 and blaTEM-1 were detected in 50% of the isolates, whereas none of the isolates harboured SHV genes. In a similar study conducted by Hyati et al. (2019) from Indonesia, Klebsiella spp. was isolated from desi chicken. Isolates that showed phenotypic resistance to ampicillin and cefotaxime were screened by PCR for blaTEM, blaSHV, blaOXA and blaCTXM genes. The isolates harboured blaSHV (9.1%), blaTEM(100%), and blaCTX-M (90.9%).
Table 2: Detection of β- lactamase genes in Enterobacteriaceae of desi chicken
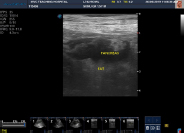
Fig. 3: Detection of Multiplex PCR II blaCTX-M Group 1 gene in Enterobacteriaceaeof desi chicken
Lane M : Molecular weight marker (100bp); Lane 1 : Known DNA standard for bla CTX-M group 1 gene (688bp); Lane 2 to 6 :Desi chicken microbiota carrying bla CTX-M group 1 gene; Lane 7 : Negative control.
Samanta et al. (2015) tested 360 poultry samples from backyard poultry and120 samples from the farmed poultry in India. Phenotypic resistant ampicillin and cefotaxime samples were screened by PCR for ESBL genes. None of the E.coli isolates from the backyard poultry harboured any ESBL gene. 29.4% of isolates from the farmed poultry were found to possess the ESBL genes. These findings are contrary to our findings in the present study.
CONCLUSION AND RECOMMENDATIONS
Even though, the desi chicken were reared in the free range system without any routine antibiotic supplementation in the feed, they found to harbour the antibiotic resistance genes which might have acquired from the environment.
These birds in turn may act as reservoirs of resistant bacteria and may play a major role in transmission of the resistant genes to other animals and humans. The results of the present study warrant the usage of antibiotics in poultry feed and suitable alternatives like probiotics, prebiotics, organic acids and synbiotics may be tried.
REFERENCES
CLSI. 2014. Clinical Laboratory Standards Institute, Performance Standards For Antimicrobial Susceptibility Testing. Twenty–Fourth Informational Supplement M 100- S24, Wayne, PA, USA.
Hasan, B., Sandegren, L., Melhus, A., Drobni, M., Hernandez, J., Waldenström, J., Alam, M. and Olsen, B. 2012. Antimicrobial drug–resistant Escherichia coli in wild birds and free-range poultry, Bangladesh. Emerg. Infect. Dis., 18(12): 2055.
Hayati, M., Indrawati, A., Mayasari, N.L.P.I., Istiyaningsih, I. and Atikah, N. 2019. Molecular detection of extended- spectrum β-lactamase-producing Klebsiellapneumoniae isolates of chicken origin from East Java, Indonesia. Vet. World, 12(4): 578-583.
Ibrahim, M.A. 2017. Antibiotic Resistant Microflora Bacteria in Intestine of Broiler Chicken. Post graduation thesis. Sudan University of Science and Technology.
Kakkar, M., Walia, K., Vong, S., Chatterjee, P. and Sharma, A. 2017. Antibiotic resistance and its containment in India. Br. Med. J., 358: 25-30.
Rao, T.S. 2009. Studies on detection of Shiga toxin producing Escherichia coli in meat and meat products by multiplex polymerized reaction and their public health significance. Ph.D. Thesis. Gadvasu, Ludhiana-141004.
Sailu, E.M., Vahjen, W. and Zentek, J. 2017. Types and prevalence of extended spectrum beta lactamase producing Enterobacteriaceae in poultry. Anim. Health Rev., 18(1): 46- 57.
Samanta, I., Joardar, S.N., Das, P.K. and Sar, T.K. 2015. Comparative possession of Shiga toxin, intimin, enterohaemolysin and major extended spectrum beta lactamase (ESBL) genes in Escherichia coli isolated from backyard and farmed poultry. Indian. J. Vet. Res, 16(1): 90.
Sharif, N.M., Sreedevi, B. and Chaitanya, R.K. 2017a. Occurrence of Beta-lactam resistant Escherichia coli among clinical cases of livestock in Andhra Pradesh. Int. J. Sci. Environ. Technol., 6(2): 1608-1615.
Sharif, N.M., Sreedevi, B., Chaitanya, R.K. and Sreenivasulu, D. 2017b. Beta-lactamase antimicrobial resistance in Klebsiella and Enterobacter species isolated from healthy and diarrheic dogs in Andhra Pradesh, India. Vet. World, 10(8): 950.